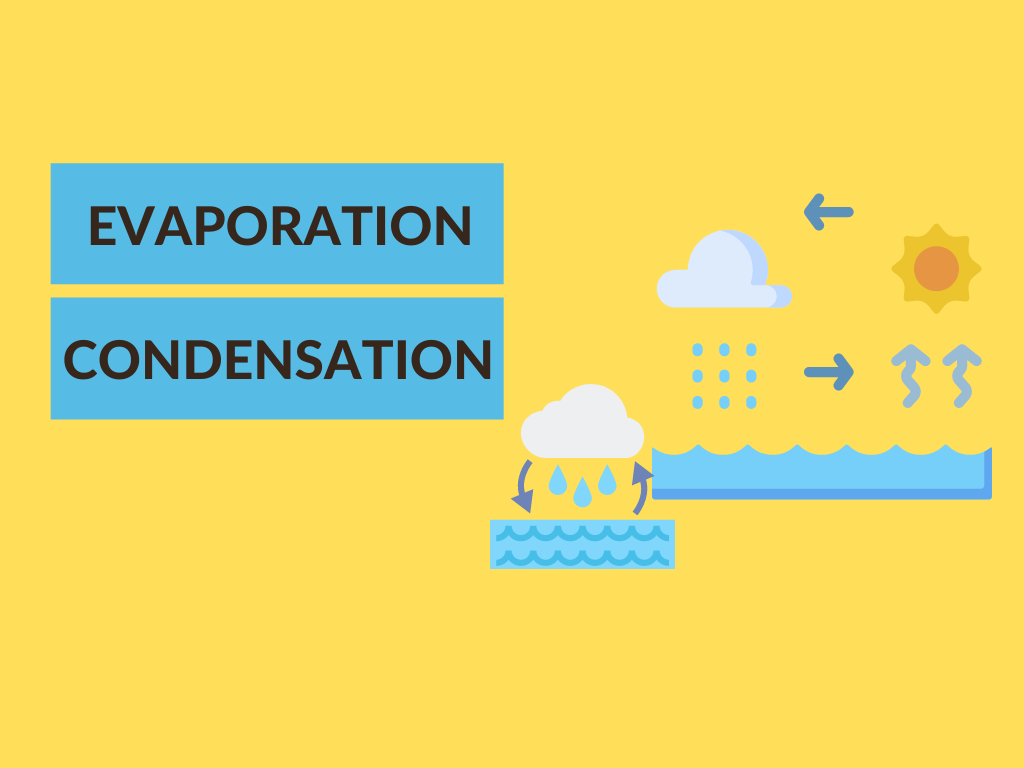
Evaporation and condensation are two of the most important physical processes for the continuous movement of water on, beneath, or above the earth’s surface.
Not to mention others who are directly or indirectly responsible for the continuous completion of the water cycle.
The primary distinction between evaporation and condensation is that evaporation is a cooling process, whereas condensation is a heating process. Condensation, on the other hand, is a warming process.
What is evaporation?
Evaporation is a type of phase transformation in which a material is converted directly from a liquid to a gaseous state. Unlike boiling, evaporation is a surface phenomenon, according to the definition.
Not to mention that the evaporation process can occur at any temperature. In fact, the rate of evaporation increases with increasing temperature. Furthermore, evaporation occurs most commonly at low altitudes.
Daily life examples of evaporation:
- Evaporation of sweat from our body
- Drying of wet floor
- Melting of ice cubes
- Drying of wet clothes
- The smoke emitted by a cup of hot coffee
What is condensation?
Condensation is a type of phase transformation in which a substance changes directly from its gaseous to a liquid state. Condensation can only occur on salt, hygroscopic nuclei-pollen grains, carbon particles, and so on, according to the definition.
This occurs prior to the gas reaching its freezing point. In fact, the higher the rate of condensation, the lower the temperature. Furthermore, condensation typically occurs at high altitudes.
Daily life examples of condensation:
- Dew on grass
- Steamy mirror in the bathroom
- Cold drinks sweat
- Fog on the windshield
- Fog in the air
Difference between Evaporation and Condensation:
| Evaporation | Condensation |
|---|---|
| Meaning | |
| Evaporation is a process where water changes into a vapor. | Condensation is the opposite process where water vapor is converted into tiny droplets of water. |
| Occurrence | |
| It occurs before a liquid reaches its boiling point. | It is a phase change regardless of the temperature. |
| Altitude place | |
| Usually takes place in low altitudes. | It happens mainly at higher altitudes. |
| Energy | |
| When evaporation takes place energy is consumed. | Here in the process of condensation energy is released. |
| Location | |
| It occurs on all surfaces, at all times, and in all places. The frequency of evaporation is when it is dry in the air, hot and windy. | When the temperature of the air decreases to the level of saturation. |
| Phase | |
| It has a phase change from liquid to gas | Here it is reversed, which means change is from gas to liquid. |
Bottom line:
As a result of the preceding discussion, Evaporation and condensation are two processes that change the state of matter from one to another. Matter exists in three states: solid, liquid, and gas. Evaporation is the process by which matter changes from a liquid to a gas. Matter changes from a gas to a liquid during condensation.
Further readings: