What is Redox Reaction?
A redox reaction is an electron transfer reaction that contains two half-reactions that are oxidation and reduction. It contains two chemical species like an oxidizing agent and a reducing agent.
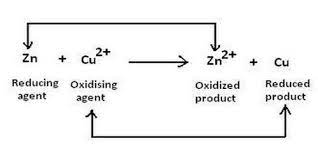
Example: Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
In the above reaction, zinc acts as an oxidizing agent whose oxidation state change from 0 to 2, and copper acts as a reducing agent whose oxidation state change from +2 to 0.
Oxidizing and Reducing Agents
Oxidizing agent: It is defined as a substance that causes oxidation by accepting electrons. In a simpler way, an oxidizing agent is a substance that gets reduced.
Reducing agent: It is defined as a substance that causes reduction by losing electrons. In a very simple way, a reducing agent is a substance that gets oxidized.

What is Oxidation?
Oxidation is the loss of electrons from a molecule or an atom or an ion which results in an increase in the oxidation state of the chemical substance. The old definitions of oxidation include loss of hydrogen or addition of oxygen.
As the loss of electrons occurs in oxidation there should be another chemical substance to accept electrons because the oxidation is known as oxidation half-reaction in the redox reaction.
Eg: 2Mg(s) + O2(g) → 2MgO(s)
Oxidation occurs in the above example by addition of oxygen to magnesium.
Eg: CH3CH2OH(aq) → CH3COOH(aq)
Oxidation occurs by removal of hydrogen in the above example. It is also an example of oxidation of alcohol group into carboxylic acid group.
Eg: Fe2+ → Fe3+ + e−
In the above example, the oxidation occurs by loss of electrons.
What is Reduction?
Reduction is the gain of electrons from a molecule or an atom or an ion which results in the decrease of the oxidation state of the chemical substance. The old definitions of reduction include a gain of hydrogen or loss of oxygen (which are opposite to oxidation). The electrons released in oxidation are gained in reduction hence in a redox reaction reduction is a half-reaction.
Eg: Ag+(aq) + e− → Ag(s)
In the above example reduction occurs by gain of electron.
Eg: 2FeO + C → 2Fe + CO2
In the above example reduction occurs by loss of oxygen.
Eg: H2 + Cl2 → 2HCl
In the above example, the reduction occurs by addition of hydrogen.
Difference Between Oxidation and Reduction
| Oxidation | Reduction |
|---|---|
| Definition | |
| Oxidation is defined as the loss of electrons from atoms or ions or molecules. | Reduction is defined as the gain of electrons from atoms or ions or molecules. |
| Other Definitions | |
| Oxidation is loss of hydrogen or gain of oxygen. | Reduction is the gain of hydrogen or loss of oxygen. |
| Oxidation State | |
| After oxidation, there is an increase in the oxidation state. | After reduction, there is a decrease in the oxidation state. |
| Charge | |
| An increase in the positive charge of chemical species is observed after oxidation. | An increase in the negative charge in chemical species is observed after reduction. |
| Chemical substance involved | |
| Oxidation occurs in reducing agents. | Reduction occurs in oxidizing agents. |
| Electron release | |
| In oxidation release of electrons into the surrounding can be observed. | In reduction taking of electrons from surroundings can be observed. |
| Energy | |
| oxidation releases energy | Reduction stores energy |
| Examples | |
| 2Mg(s) + O2(g) → 2MgO(s) | Ag+(aq) + e− → Ag(s) |
Conclusion
Both oxidation and reduction are half-reactions of a redox reaction. The major difference between oxidation and reduction is that one will lose electrons (oxidation) and the other will gain electrons (reduction).


