SN1 and SN2 reactions are two nucleophile substitution reactions in which SN1 involves only one molecule whereas SN2 reaction involves two molecules.
What is A Nucleophile?
A nucleophile is an atom or molecule or any functional group that has electron pairs to donate. It is negatively charged due to unused electrons.
What is A Nucleophilic Substitution Reaction?
The reaction which involves the substitution of an atom or a functional group with a negatively charged atom or functional group that contains unused electrons (nucleophile) is known as nucleophilic substitution reaction. There are two types of nucleophilic substitution reactions. They are SN1 and SN2.
What is An SN1 reaction?
SN1 reaction is a unimolecular nucleophilic substitution reaction which occurs in two steps.
step 1: Ionization of alkyl halide in presence of aqueous acetone. It produces a carbocation as an intermediate.
Step 2: Attack of nucleophile: The nucleophile will attack the carbocation which results in a mixture of enantiomers as a product.
An example of the SN1 reaction is the hydrolysis of tertiary butyl bromide to form tertiary butanol.
Mechanism of The SN1 Reaction
- Step-1: Ionization of alkyl halide in presence of aqueous acetone leads to the formation of a tertiary butyl carbocation by separation of a leaving group (a bromide anion) from the carbon atom. This is a slow step.
- step-2: Attack of nucleophile: The nucleophile will attack the carbocation. If the nucleophile is a neutral molecule (i.e. solvent) a third step is required to complete the reaction. When the solvent is water, the intermediate is an oxonium ion. This is a fast step.
- Step-3: Removal of a proton on the protonated nucleophile by water acting as a base, forming the alcohol and a hydronium ion. This is also a fast step.
SN1 reactions can also be known as dissociative mechanisms. The reaction does not depend on the strength of the nucleophile and follows first-order kinetics.
What is An SN2 Reaction?
The SN2 reaction is a bimolecular nucleophilic substitution reaction that occurs in one step. The breaking of the C-X bond in the formation of a new bond occurs simultaneously through a transition state.
An example of the SN2 reaction is the attack of bromide ion (the nucleophile) on ethyl chloride results in ethyl bromide, by separating chloride ion as the leaving group.
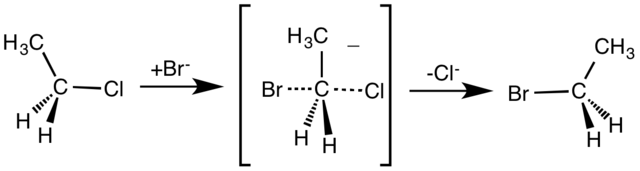

The attack of nucleophile occurs from the backside hence inversion of configuration or Walden inversion can be seen. The rate of an SN2 reaction is second order as the rate-determining step depends on the concentration of nucleophiles as well as on the concentration of substrate. SN2 is an associative mechanism.
Difference Between SN1 and SN2 Reactions
| SN1 reaction | SN2 reaction |
|---|---|
| SN1 is a unimolecular reaction | SN2 is a bimolecular reaction |
| SN1 follows first-order kinetics | SN2 follows second order kinetics |
| SN1 occurs in two steps | SN2 occurs in one step |
| The rate of reaction depends on the concentration of the substrate. | the rate of reaction depends on the concentration of both the substrate and the nucleophile |
| formation of intermediate occurs in the SN1 reaction | No intermediate formation occurs in the SN2 reaction |
| As the leaving group leaves, the substrate forms a carbocation intermediate followed by a nucleophilic attack. | The reaction occurs through a single transition state. |
| A racemic mixture of products are formed in the SN1 reaction. | Inversion of configuration occurs in the SN2 reaction. |
| polar protic solvents (which donates a hydrogen ion readily) are used in an SN1 reaction | polar aprotic solvents are used in an SN2 reaction |
| SN1 follows the dissociative mechanism | SN2 follows the associative mechanism |
| The SN1 reaction is mostly favored by tertiary alkyl halides | The SN2 reaction is mostly favored by primary alkyl halides. |
Conclusion
SN1 and SN2 are two types of nucleophilic substitution reactions. Both these reactions are different in many aspects. SN1 is a unimolecular reaction and SN2 is a bimolecular reaction. The mechanisms of SN1 and SN2 are also different by producing an intermediate in SN1 and no intermediate is produced in the SN2 reaction.