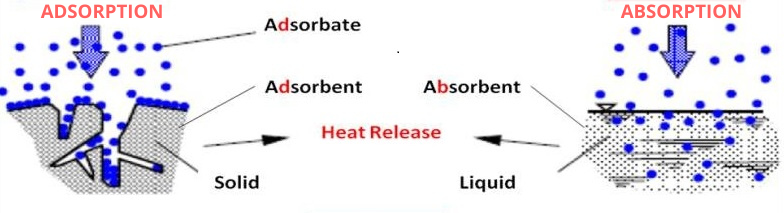
What is Absorption?
It is a physical or chemical process in which atoms or ions or molecules get incorporated into a bulk phase like liquid or solid. It is a bulk phenomenon. The substance which penetrates is absorbate and the bulk space which gives space for absorbate is absorbent. An example of absorption is soaking up spilled milk with a paper towel. The paper travel absorbs the milk.
Types of Absorption
There are two types of absorption
- Chemical absorption
- Physical absorption
Chemical absorption: It is also known as reactive absorption. It is a chemical reaction between the absorbate and absorbent. It depends on the stoichiometry of the reaction and the concentration of its reactants.
Physical absorption: It is a non-reactive process. An example is the absorption of oxygen present in the air by water. It depends mainly on physical properties like solubility, pressure, and temperature.
What is Adsorption?
Adsorption is a surface phenomenon in which particles (ions or molecules or atoms or electrons) are attached to the top layer or surface of a material. The substance which deposits on the surface is called an adsorbate (H2, N2, O2 gases). The substance which provides a surface for adsorption is called an adsorbent (silica gel, zeolites).
Types of Adsorption
Adsorption is mostly of two types.
- Physical adsorption
- Chemical adsorption
Physical adsorption: It is also known as physisorption. It occurs due to week Vanderwaal forces between adsorbate and adsorbent. It is multilayered and not specific.
Chemical adsorption: It is also known as chemisorption. It occurs due to strong chemical bonding forces between adsorbate and adsorbent. It is single-layered and highly specific.
Difference Between Absorption and Adsorption

| Absorption | Adsorption |
|---|---|
| Definition | |
| The incorporation of a substance into another substance is generally known as absorption. | The deposition of a substance on the surface of another substance is called adsorption. |
| Phenomenon | |
| Absorption is a bulk phenomenon. | Adsorption is a surface phenomenon. |
| Process | |
| Absorption is a nonspontaneous process. | Adsorption is a spontaneous process. |
| Molecular interaction | |
| In absorption, greater molecular interaction occurs. | In adsorption, less molecular interaction occurs. |
| Temperature | |
| Absorption is not affected by temperature. | Adsorption is favored by low temperatures. |
| Heat exchange | |
| Absorption is an endothermic reaction. | Adsorption is an exothermic reaction. |
| Concentration | |
| Absorption is same throughout the absorbent. | In adsorption concentration on the surface is different from that of adsorbent. |
| Rate | |
| It occurs at a uniform rate. | Adsorption increases steadily and reaches equilibrium. |
| Substances involved | |
| Absorbate and absorbent are involved in absorption. | Adsorbate and adsorbent are used in adsorption. |
Conclusion
Absorption and adsorption are two different phenomena. The absorption and adsorption both are Physico-chemical processes in which a small quantity of one substance enters or gets attached to another substance of higher quantity.
Uses of Absorption
Absorption is used in absorption chillers for space cooling applications. It is also used in the clean-burning of fuels. The process of gas absorption by a liquid is used in the hydrogenation of oils. It is also used in carbonation.
Uses of Adsorption
Adsorption is used in the treatment of diarrhea. This phenomenon can be seen in a gas mask in which the obnoxious gases are absorbed on the surface of the mask. It is used in the clarification of sugar, in the paint industry, and also in the adsorption of excessive gas in the gastrointestinal tract.




